Carbonyl Mechanisms: Elimination (1,2-Elimination)
Carbonyl Mechanisms: Elimination (1,2-Elimination)
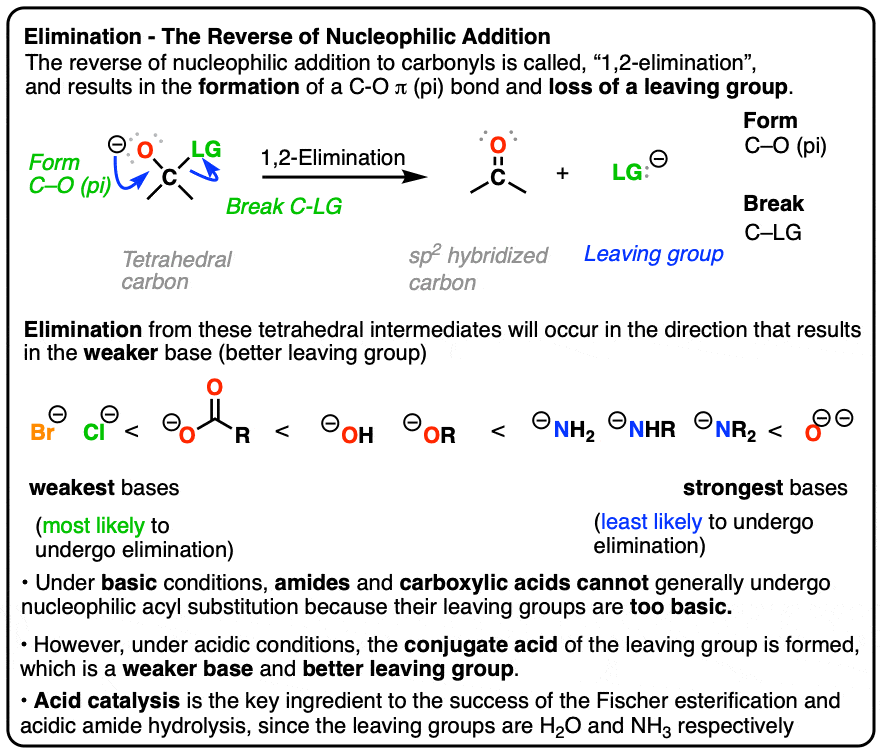
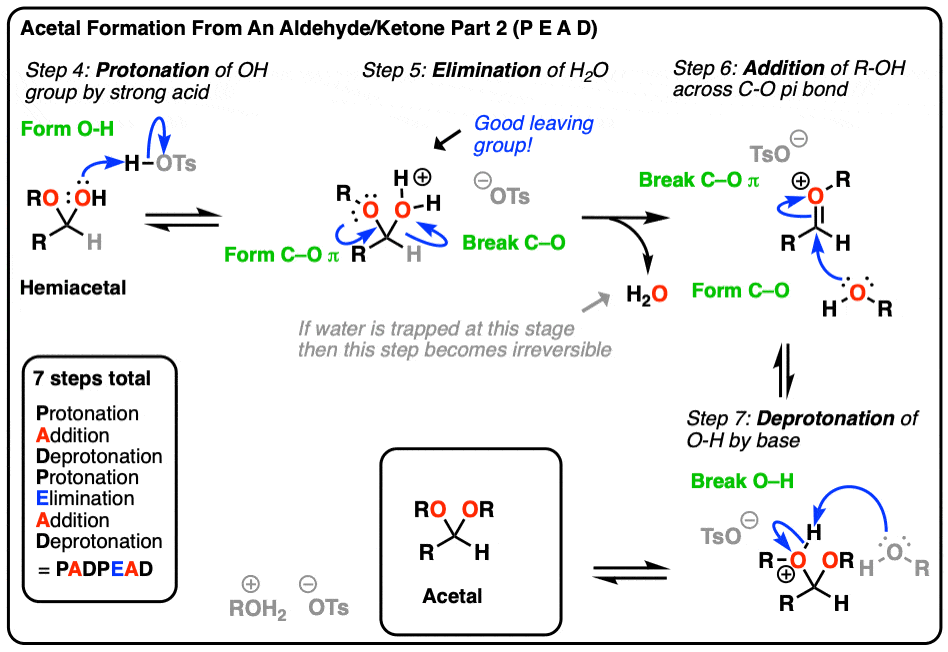
If the [1,2] addition is the most important mechanism for carbonyls, then the #2 most important reaction has got to be [1,2]-elimination. This step is just the [1,2]-addition in reverse, except you're expelling a leaving group from a tetrahedral carbon to give you back a new π bond (i.e. a carbonyl).

Elimination Reactions
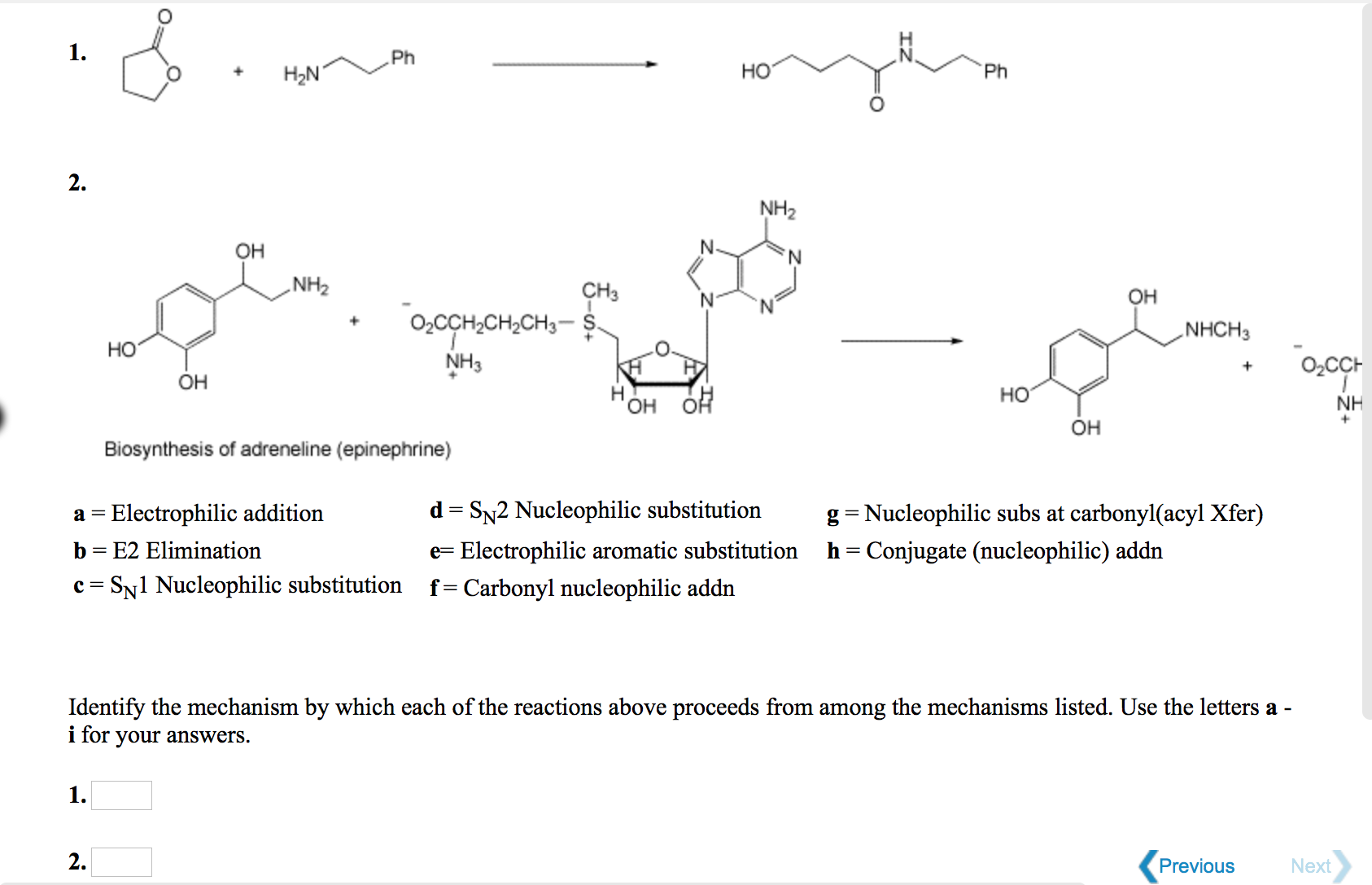
Solved a = Electrophilic addition b = E2 Elimination c = SN1

Elimination reaction

Palladium-catalyzed allene synthesis enabled by β-hydrogen elimination from sp2-carbon
Carbonyl Mechanisms: Elimination (1,2-Elimination)

Carbonyl Mechanisms: Elimination (1,2-Elimination)

Beta-Elimination - an overview

Elimination reactions in halogenoalkanes - Crunch Chemistry
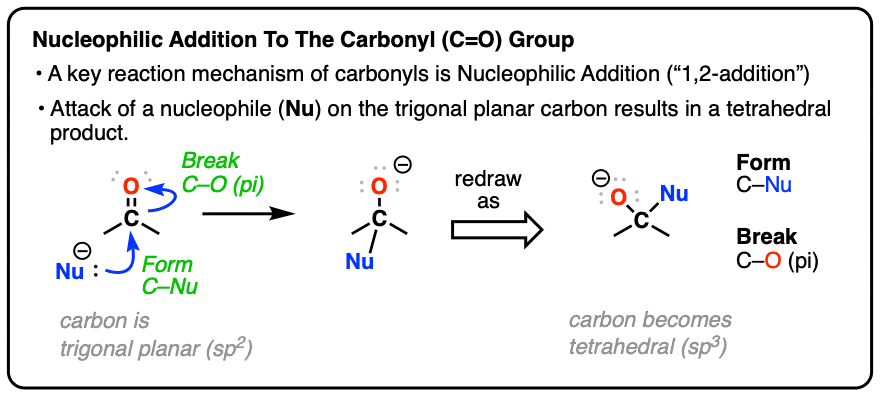
Carbonyl Mechanisms: Elimination (1,2-Elimination)
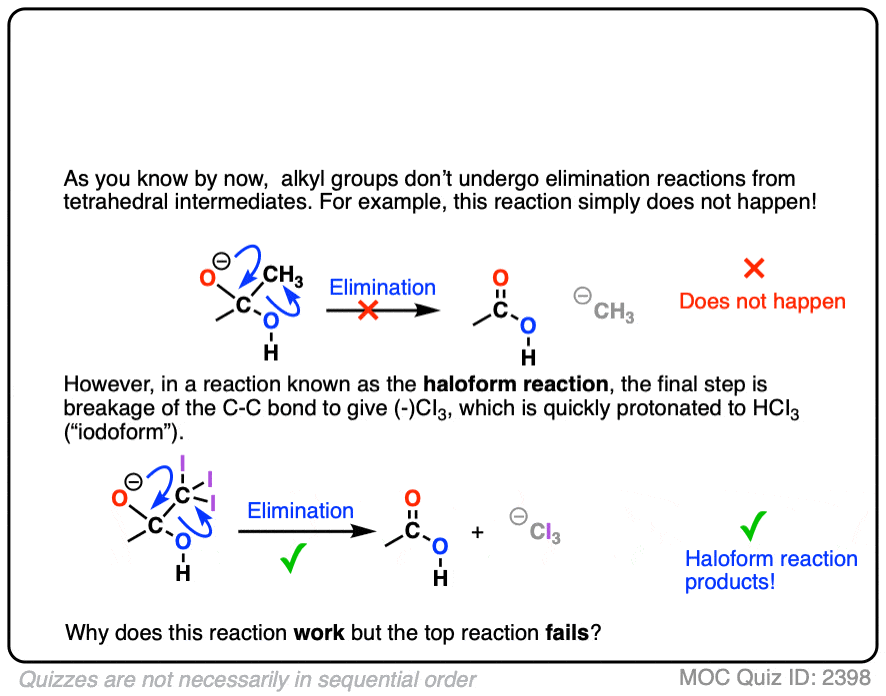
Carbonyl Mechanisms: Elimination (1,2-Elimination)